
#1 Best Selling IGNOU CBCS Assignments in All Available in Market
Bought By: 4766 Students
Rating: 3.8
Get IGNOU BCHCT-131 Assignments Soft Copy ready for Download in PDF for (January 2024 - December 2024) in English Language.
Are you looking to download a PDF soft copy of the Solved Assignment BCHCT-131 - Atomic Structure, Bonding, General Organic Chemistry and Aliphatic Hydrocarbons? Then GullyBaba is the right place for you. We have the Assignment available in English language.
This particular Assignment references the syllabus chosen for the subject of Chemistry, for the January 2024 - December 2024 session. The code for the assignment is BCHCT-131 and it is often used by students who are enrolled in the BSC (Honours), BSCG Degree.
Once students have paid for the Assignment, they can Instantly Download to their PC, Laptop or Mobile Devices in soft copy as a PDF format. After studying the contents of this Assignment, students will have a better grasp of the subject and will be able to prepare for their upcoming tests.
PART-(A)
1. Using a suitable diagram explain the spectral transitions between different energy levels of hydrogen atom. Also name these series of lines and give the region of electromagnetic radiation in which they appear.
2. What was the purpose of Davisson and Germer experiment? Explains and analyse its results.
3. (a) What is a well-behaved wave function? Illustrate using suitable diagram.
(b) Give the significance of ψ and ψ2.
4. What are different quantum numbers? Explain their significance.
5. Briefly explain the following:
(i) The aufbau principle
(ii) Hund’s rule
(iii) Pauli exclusion principle
6. a) Arrange the following compounds in order of decreasing lattice energy: LiF,MgO,KBr. Justify your answer.
b) Predict coordination number of the cation in crystals of the following compounds.
MgO: if ionic radii for Mg2+ =65 pm and O2– = 140 pm.
MgS: if ionic radii for Mg2+ =65 pm and O2– = 184 pm.
7. Why does the bond length decrease in the case of multiple bond formation? Explain with the help of an example. Also explain why a multiple bond is stronger than a single bond.
8. a) The observed dipole moment of HI is 0.38 D. Calculate the percentage ionic character of the bonding HI if bond distance is 161 pm
b) Melting point of aluminum fluoride is higher than the melting point of aluminum iodide. Explain
9. Draw the resonance structures of carbon monoxide. Also give the electronic configuration of the combining atoms
10. Draw the energy level diagram for carbon monoxide molecule. Write its molecular orbitals configuration and calculate its bond order. Comment on its magnetic behaviour.
PART-(B)
11. Draw all the stereoisomers of 2-bromo-3-chlorobutane and classify them as enantiomers and diastereoisomers.
12. (a) What are resolving agents? Give examples of three acidic and three basic resolving agents.
(b) Write the Fischer projection for the molecule.

13. Draw and explain the energy propile for ring flipping of chair conformation of cyclohexane.
14. Arrange the following carbocations in the increasing order of stability and explain the reason for your answer:
A primary carbocation, a tertiary carbocation, a secondary carbocation.
15. Arrange the following nucleophiles in the increasing order of their strength and give reason for your answer.
CH3-, NH2-, CN-, OH-, I-
16. (a) Define octane number. How does the octane number of a hydrocarbon vary with the following?
(i) Branching of the hydrocarbon chain
(ii) Decrease in the chain length
(iii) Unsaturation
(b) How would you synthesise hexane using Wurtz reaction. Explain giving equation.
17. How would you prepare an alkene using Wittig reaction? Explain the mechanism also.
18. What is Markownikoff’s rule? Explain using this rule why 2-bromopropane is the major product of bromination of propene.
19. Discuss different methods of preparation of propyne.
20. Explain whether the following compounds are aromatic or not?

PART-(A)
1. Derive the following expression for the energy of electron in the nth orbit.
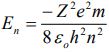
2. What is a black body? Discuss the important features of black body radiation giving a suitable diagram.
3. Derive the time independent Schrodinger equation for a particle.
4. Draw the shapes of 5d orbitals. Also indicate the signs of wave functions and nodal planes in the diagram.
5. Write the electronic configurations for the following elements.
(i) Cr
(ii) Mo
(iii) Ag
Also explain your answer.
6. (a) Write down Born-Haber cycle for CaCl2 formation.
(b) Discuss the factors which affect the solubilities of ionic solids in water.
7. Write the assumptions of calculating the formal charge in a molecule. Calculate the formal charge in CH3-C=O-CH3.
8. Draw the resonance structures of HCl. Out of them which one has little importance as a resonance structure and why?
9. (a) Calculate the percentage ionic character in HBr gas.
Use following data:
The dipole moment of HBr = 2.635 x 10-30 C m
Bond length of HBr = 141 pm.
(b) An anion will be more easily polarized, if it is large and highly negatively charged. Explain using suitable examples.
10. Draw and explain the molecular orbitals formed by the linear combination of following atomic orbitals.
(i) px and px orbitals
(ii) py and py orbitals
PART-(B)
11. Explain the following giving suitable examples:
(i) Position isomerism
(ii) Functional group isomerism
(iii) Chiral centre
12. (a) Write two more Fischer projection formulas for the following compound:
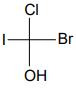
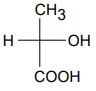
(b) Write the enantiomer of the following compound and assign their configurations as R or S.
13. Draw the possible conformations of cyclohexane and explain their relative stability.
14. (a) Arrange the following compounds in the increasing order of their acidities and give reason in support of your answer.
3-chlorobutanoic acid, butanoic acid, 4-chlorobutanoic acid and 2-chlorobutanoic.
(b) Write acid the resonance structures of propanoate ion.
15. What is pKa? How does it help in explaining the basicity of different nucleophiles? Write a nucleophilic reaction indicating the nucleophile and its conjugate acid.
16. (a) How would you prepare cyclopentane starting from barium adipate? Write the reactions involved.
(b) Explain the term pyrolysis and cracking giving suitable examples.
17. (a) Explain Saytzeff rule giving a suitable example.
(b) Explain the mechanism of Birch reduction.
18. (a) How would you prepare 2-propanol from propene? Write the steps involved in the conversion.
(b) Write the products of ozonolysis of 2-methyl-2-butene.
19. Give the mechanism of hydration of ethyne and the product form.
20. Giving suitable diagrams, explain the structure of benzene.
The IGNOU open learning format requires students to submit study Assignments. Here is the final end date of the submission of this particular assignment according to the university calendar.
Here are the PDF files that you can Download for this Assignment. You can pick the language of your choice and see other relevant information such as the Session, File Size and Format.
In this section you can find other relevant information related to the Assignment you are looking at. It will give you an idea of what to expect when downloading a PDF soft copy from GullyBaba.
In addition to this Assignment, there are also other Assignments related to the BSCG Chemistry you are preparing for. Here we have listed other Assignments that were bought along with this one.